
This product is no longer available for sale in Europe. On the other hand, you can find all the consumable products associated with your devices in our catalog.
→ Référence : Appareil de diagnostic médical V2 : EXS1SDP2001
The direct measurement of chloride ions by the electrochemical electrode is expressed in mini moles and allows immediate diagnosis by the practitioner:
– Calibration and self-test procedure
– Non-displacement and concentration of sweat by Exsupatch®
– Direct reading of chloride ions by the selective probe
– Specialized, secure calculator, electronically managing the data extracted via USB port
– Guaranteeing the reliability and reproducibility of test results
The Exsudose® test can be performed on all newborns weighing 3-5 kg and 3 weeks old as well as on all children or young adults presenting symptoms associated with cystic fibrosis.
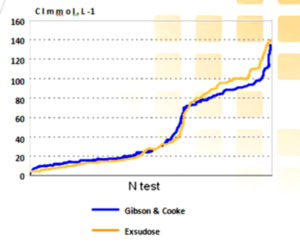
Comparison of the results of the different methods:
ELECTROLYTE BOTTLE
– A 60 ml bottle of electrolyte – A pouring cap
Kit : EXS1SDP2018V1
Lot : EXS1SDP2021V1
SWEAT KIT
– 20 “pilocarpine” tampons in blister
– 20 Exsupatch®
– Disposable handling forceps
Kit : EXS1SDP2018V1
Lot : EXS1SDP2021V1
CALIBRATION KIT
– 1 bottle solution A (20meq)
– 1 vial solution B (60meq)
– 1 vial solution C (100meq)
– 20 “pilocarpine” tampons in blister
– 20 Exsupatch®
– Disposable handling forceps
Kit : EXS1SDP2035V1
Lot : EXS1SDP2034V1
CUPS KIT
– A bag of 60 cups
Kit : EXS1SDP2016V1
Lot : EXS1SDP2014V1
MICROSTIM®
– A Microstim® device
Kit : EXS1SDP2060V1
Lot : EXS1SDP2059V1
SOLUTION KIT
INTERNAL CONTROLS
– 1 bottle solution 1 (40meq)
– 1 vial solution 2 (80meq)
Kit : EXS1SDPSOLCONTR
Lot : EXS1SDPSOLC5
Referring doctor for Exsudose®: Martine MARCHAND – Department of Biochemistry – Hormonology, CHU Robert Debré AP-HP, PARIS.
M. Marchand, C. Jarreau, M. Chauffert, I. Garcia, D. Asselin, J-P. Thouvenot, A-F. Genest – The sweat test. ABC 1998 Vol.56, 2: 215-21.
M. Marchand, Gersende Flament – EXSUDOSE® Biochemistry RDB Expertise – May 2001. M. Marchand, Bichr Allaf – EXSUDOSE® Biochemistry RDB Expertise – July 2007.
Hôpital Robert Debré, C.H.U. Nantes, C.H.R.U. Tours, C.H. Dunkerque, C.H.U. Dijon, C.H.U. Nice, C.H. Libourne, Hôpital Armand Trousseau, C.H.U. Bordeaux, C.H.U. Rennes, C.H.U. Besançon, C.H.U. Nancy, C.H. Versailles, Hôpital Ambroise Paré, C.H.U. Besançon, C.H.I. Poissy St Germain, C.H.U. Toulouse, C.H. Sud-Francilien, G.H.I. Le Raincy-Montfermeil.